
Single-Use Virus Filtration System
Next-level flexibility to meet your unique bioprocess needs.

Brand-agnostic design allows the use of best-in-class components for every part of the system

Integrate with your preferred virus filter brand to provide highly reliable virus retention

Dynamic flow control automatically decreases flow rate should filters foul and pressure increase, providing safe working conditions

Manual or fully automated control; can be integrated with DeltaV®, Rockwell Automation® or other platfom

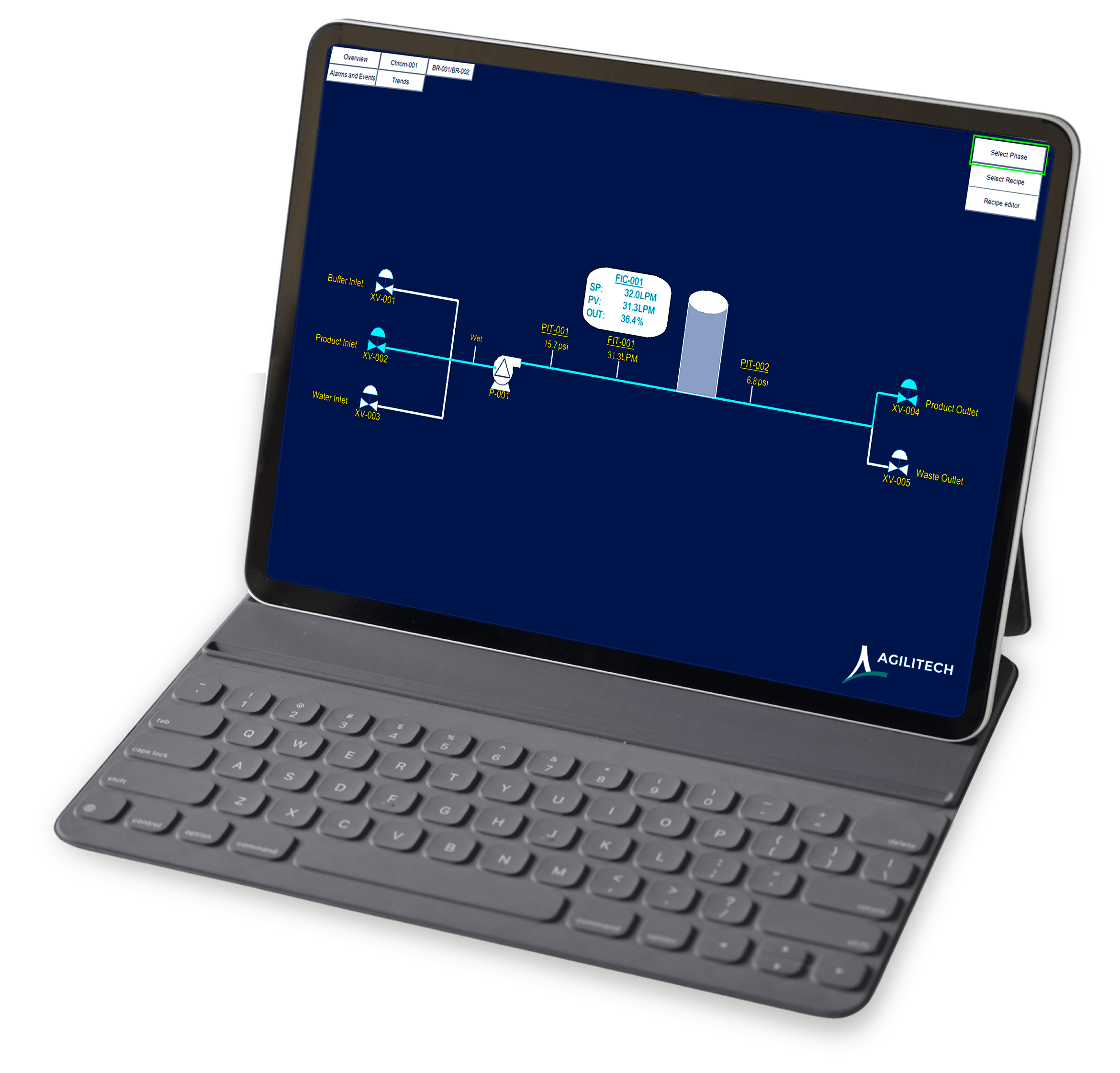
Volume totalizer monitors liquid volume pumped through the system

Multiple inlets (for product, buffer, water) and outlets (for product collection or waste)
Want to learn more about the Agilitech single-use virus filtration system?
A single-use virus filtration system that redefines flexibility
Agilitech provides solutions for every step of the bioprocess, including cell culture purification to ensure product safety. The Agilitech virus filtration system is a highly versatile single-use platform that can be tailored to your unique needs; for example, it can be adapted to accommodate your preferred virus filter brand, offering next-level flexibility.
As with all Agilitech single-use technologies, our customers are co-designers of their solution. We ask the right questions and take the time to understand not only what you’re doing now, but also where you’re going before adapting our standard virus filtration system to fit your bioprocess requirements. Our brand-agnostic design is truly unique and provides complete flexibility to meet your exact process requirements, while every part of the system features best-in-class components to optimize performance.
Flexible control
Operate via manual control or easily integrate with your preferred automation and control system.
The intuitive manual control panel is easy to use. Our engineers have listened to customer needs and have identified the functions and data that operators need to access most often; the user interface brings these functions and data to the top layer of the screen to eliminate endless clicking or digging for critical information.
The virus filtration system can also be delivered with your preferred automation and control platform. Our engineering experts have years of experience with DeltaV®, Rockwell Automation®, and other leading platforms; we will integrate the system to meet your requirements. Regardless of the platform you choose, the user experience is the same due to the ISA-88 batch-compliant structure.
A choice of models
A range of models is offered to accommodate different tubing sizes and flow rates for lab to production scale bioprocesses. All models provide the same basic functionality and performance.
F500
Tubing ID:
0.50 in
Flow Rates:
0.167–15 LPM
Flow Sensor
Performance:
0–20 LPM (± 3%)
F750
Tubing ID:
0.75 in
Flow Rates:
0.167–20 LPM
Flow Sensor
Performance:
0–50 LPM (± 3%)
F1000
Tubing ID:
1.0 in
Flow Rates:
0.8-90 LPM
Flow Sensor
Performance:
0–100 LPM (± 3%)
Technical Specifications
| Attribute | Specification |
|---|---|
| Pressure | 0-60 psi/4 bar |
| Operating Temperature Range | 2–50°C |
| Humidity (non-condensing) | 10–90% |
|
Utility Required, Skid Power*
|
110-120 VAC (F500/F750) 200-230 VAC (F1000) |
| Wetted Materials | USP <88> Class VI and animal free |
| Inlets | 3 |
| Outlets | 2 |
| Flow Accuracy | ±3% |
| Pressure Sensor Performance | 0–6 psi (± 2%) 6–30 psi (± 3%) 30–60 psi (± 5%) |
*Compressed air (oil free) @ 80 psi/5.5 bar
Specifications represent standard Agilitech models and may change when customized for specific customer applications. Specifications subject to change without notice.

Quality manufacturing backed by world-class service and support
All Agilitech single-use systems are manufactured to the highest standards and delivered with the documentation you need:
- Standard Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) documentation provided upon delivery; we can also use your specific documentation, if preferred
- Installation Qualification (IQ) and Operational Qualification (OQ) testing and documentation available for use in cGMP production
All Agilitech single-use systems are also delivered with a recommended preventative maintenance plan and spare parts list. We also provide maintenance service to ensure your system operates at peak performance.
Related Content
DOWNSTREAM COLUMN BLOG: Flexible Single-Use Technology that Adapts to an Evolving Industry
One thing constant in biopharmaceutical manufacturing is change. There is an ever-present need to adapt to new therapeutic modalities, more cost-effective approaches, higher product demands, and even a worldwide pandemic.
PODCAST: Addressing the increasing demand for single-use technologies and supply chain shortages with future-proof systems
We’re pleased to share this new podcast recently published by Downstream Column; a conversation about single-use technologies.
PODCAST: Single-Use Mixers – Ensuring the Customization, Scalability and Supply Required for Success
In this podcast, Brandy Sargent, Editor at Cell Culture Dish, talks with Phil Sanders (Chief Innovation Officer) and Dennis Hodgson (Director, Biotech) from Agilitech about the benefits of single-use mixers, dealing with supply chain concerns, ensuring scalability, and tailoring a mixer to meet specific process needs.
Want to learn more about Agilitech single-use virus filtration systems?
Please complete this form and an Agilitech specialist will be in touch.



