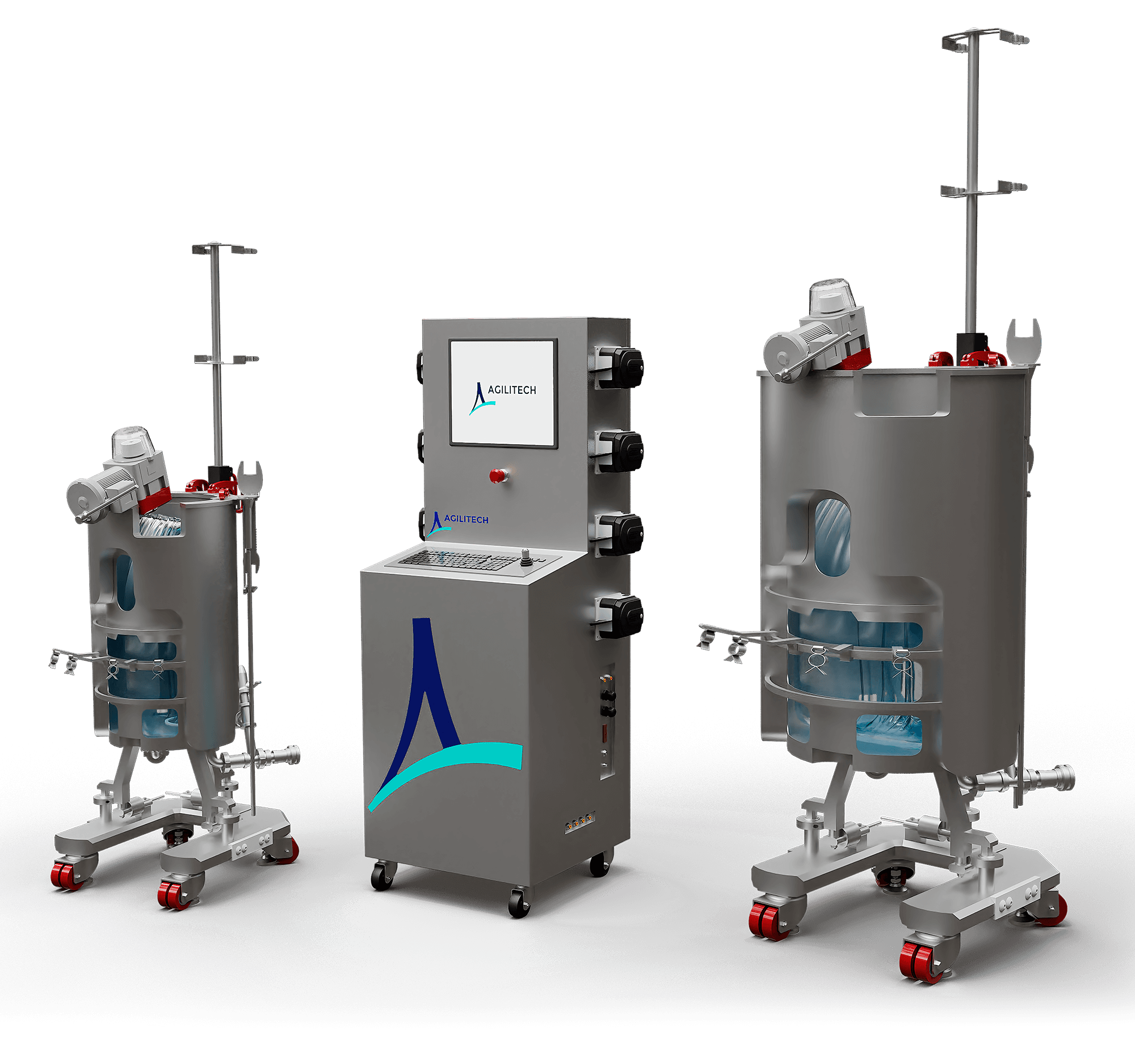
Flexible Bioprocess Controller for Single-Use Bioreactors Up To 2000 L
Adapts to your changing needs

Brand-agnostic design for use with any brand single-use bioreactor

Capable of simultaneous operation of two different single-use bioreactor brands, as well as different size bioreactors

Delivered with your preferred state-of-the-art open industrial automation and control platform, whether that's DeltaV®, Rockwell Automation®, or another platform

Up to 8 mass flow controllers with variable ranges for low flow (0-50 SLPM) and high flow (0-2500 SLPM)

Up to 8 feed pumps with variable ranges for low flow (1-1750 mL/min) and high flow (0-3500 mL/min)

Capable of connecting standard pH/DO probes or using optical patches

Integrated RTD with options for temperature control via TCU

Integrated exhaust filter heaters
Compatible with any brand single-use bioreactor
The Agilitech large-scale bioprocess controller provides simultaneous control of up to two single-use bioreactors from 30 L up to 2000 L. A unique brand-agnostic design allows for integration with any brand single-use bioreactor.
ISA-88 standards maximize flexibility
Controls are based on an ISA-88 batch-compliant structure, which provides you the flexibility to use the bioreactor controller as a stand-alone system or part of a larger unified system architecture. Since the building blocks for automation are already in place, you can start out using the system with local control and then integrate with a larger system when you’re ready. The controller allows for batch and recipe control and offers MES capability with little or no software modifications.
No proprietary software puts you in control
The bioprocess controller can be adapted to any modern automation and control platform—whether that’s DeltaV®, Rockwell Automation®, or another platform. And we never lock down the system, which enables you to make modifications on your own.
Eliminate “islands of automation” in your current process, while future-proofing for what comes next
Many bioprocessing manufacturing sites have “islands of automation” where standalone equipment is controlled locally or several pieces of equipment from the same manufacturer are connected with each other. Therefore, there is no single unified platform that controls the entire bioprocess, resulting in “islands of automation” with different levels of control that are disconnected from the rest of the process. This leads to issues with data silos, data integrity and analyses, as well as costly workarounds or manual work to bridge the gap. The end result is an inefficient overall process that continues to get worse over time. Since Agilitech bioprocess controls are based on an ISA-88 batch-compliant structure, and we use open industrial platforms and not proprietary software, we can integrate your existing equipment into a single unified platform.
Technical Specifications
| Attribute | Controller |
| Dimensions | 65 x 24 x 27.75 in (H x W x D) |
| Operating Temp. | 5 – 40°C |
| Relative Humidity | 5 – 95% |
| Agitation | Single-Use Bioreactor |
| pH/DO | Presense for optical sensors – capable of standard pH/DO probes |
| Temp | 100 ohm platinum |
| Attribute | Controller |
| Liquid Control | Up to 8 feed pumps with variable ranges from low flow (1-1750 mL/min) to high flow (0-3500 mL/min) |
| Temperature Control | via TCU |
| Vent Filter Heater | Integrated exhaust filter heater |
| Gas Control | Up to 8 MFCs with variable ranges from low flow (0-50 SLPM) to high flow (0-2500 SLPM) |
| Single-Use Vessels | 30 – 2000 L single-use bioreactor |
DeltaV is a registered trademark of Emerson; Rockwell Automation is a registered trademark of Rockwell Automation, Inc.

Quality manufacturing backed by world-class service and support
All Agilitech products are manufactured to the highest standards and delivered with the documentation you need:
- Standard Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) documentation provided upon delivery; we can also use your specific documentation, if preferred
- Installation Qualification (IQ) and Operational Qualification (OQ) testing and documentation available for use in cGMP production
Agilitech bioprocess controllers are also delivered with a recommended preventative maintenance plan and spare parts list. We also provide maintenance service to ensure your system is always operating at peak performance.
You have a vision for your process.
We can help you get there.

