Biotech Engineering Services
A full range of services to help you optimize and manage your bioprocess
Bioprocess engineering services for biotech research labs through full-scale cGMP manufacturing
Agilitech provides a wide range of engineering services for bioprocess design and implementation, as well as existing bioprocess optimization. Our services include:
Integration Services
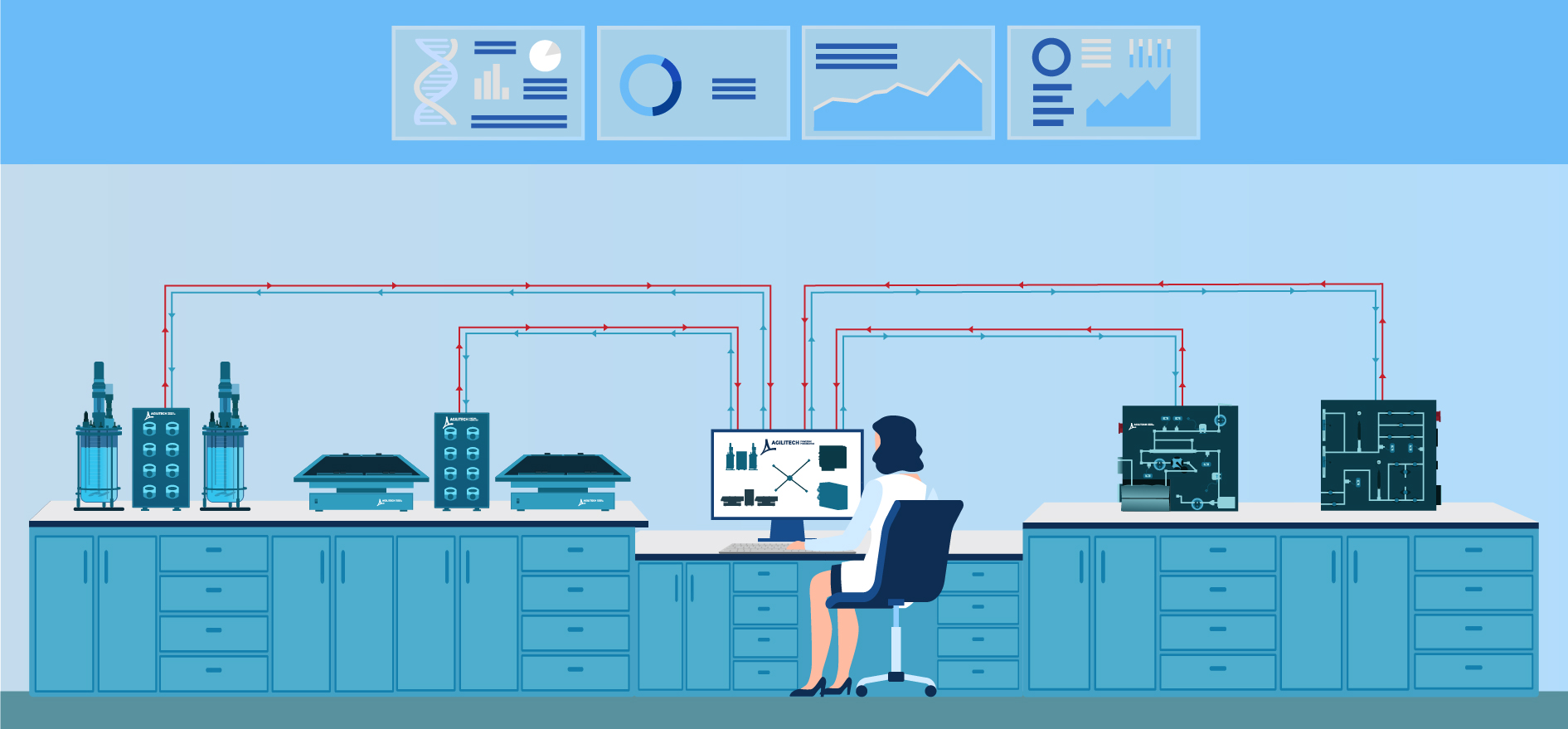
Integrate stand-alone lab equipment into a single system to improve data integrity and drive operational efficiency
Too often, laboratories become overwhelmed with a variety of stand-alone equipment that is needed for specific tests and assays, while concurrently conducting process development and characterization studies. Disparate equipment makes it cumbersome to access and analyze data, which can negatively impact decisions about your process.
Agilitech has specialized expertise integrating equipment from different manufacturers into a single system, with flexible automation and centralized control, which makes it easier to access and analyze data. Users can display data the way they want to see it, look back at past tests, build reports and evaluate trends that enable more informed decision-making about their process.

Bioprocess Automation Engineering and Implementation
Maximize process efficiency and minimize risk
Our team of expert engineers applies years of experience in both bioprocess engineering and automation to design and implement state-of-the-art bioprocess automation solutions that maximize operational efficiencies and minimize risk. We work closely with you to design and implement a flexible automation platform and management information system that is tailored to your current process and easily optimized as your needs change. Our depth of experience enables us to support virtually any leading platform, whether you prefer DeltaV, Rockwell Automation, Wonderware or other platform.
Process Optimization
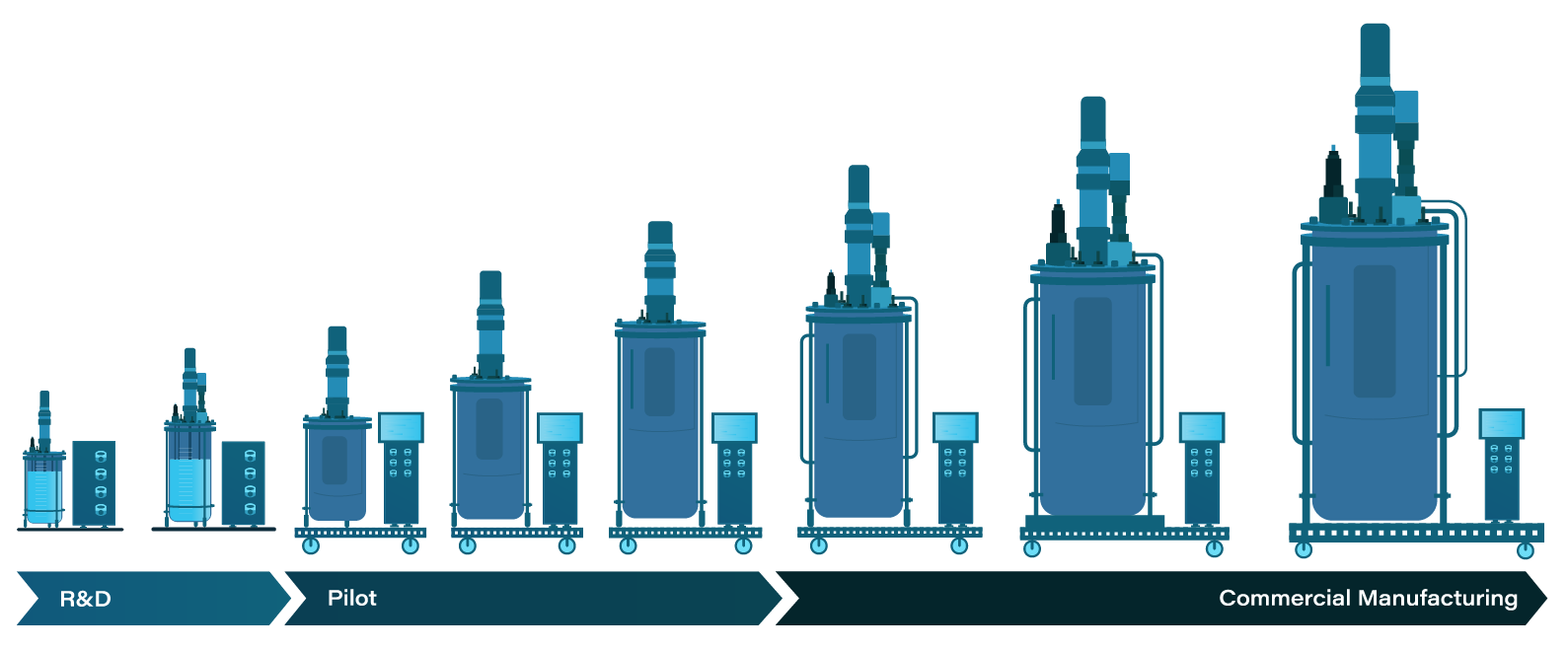
Improve your bioprocess from early phase development through scale-up and commercial manufacturing
Virtually every biotech lab and production site has pain points and trouble spots that can be optimized to increase operational efficiencies. Agilitech can help you pinpoint these trouble areas and work with you to optimize your process.
The Agilitech team has many years of experience in both biotech processing and production methods, and automation, as well as mechanical and electrical engineering. Engage our team to speak with end users in your lab and/or production environment to diagnose bottlenecks or other issues. We will assemble the right team of engineers to analyze your process and pain points, and deliver solutions to improve your process.

Commissioning and Qualification Services
Make a smooth transition from a non-GMP to cGMP environment
Transitioning to a cGMP manufacturing environment requires diligent preparation and extensive documentation. Agilitech can facilitate your move to cGMP manufacturer, for a smooth transition.
Our team of qualification engineers has years of experience with commissioning and qualification activities. We can provide support with the procurement of new equipment and perform commissioning and qualification activities to ensure the equipment is ready for production. Whether you are building a full manufacturing facility from the ground up or need help adding a single skid to your existing facility, Agilitech can help.
Staff Augmentation
Add talent and expand your team as you grow
Is your manufacturing capacity growing at a rapid rate? Does your automation team need to off-load everyday troubleshooting to focus on longer term projects? Agilitech can help you keep your process up and running during periods of rapid growth. Highly experienced and knowledgeable Agilitech automation engineers are available to support your needs. With broad experience in multiple automation control environments, such as Emerson DeltaV, Rockwell FactoryTalk, Wonderware, and more, we can fill the resource gaps to minimize downtime from automation issues.