As featured in Downstream Column:
Multipurpose Single-Use Filtration Systems
One product configurable for multiple bioprocessing steps: depth filtration, virus filtration, and sterile filtration.

One product, multiple applications.
Flexible 3-in-1 design can be adapted to virtually any external filtration system for depth filtration, virus filtration, and sterile filtration.
Agilitech’s highly versatile multipurpose single-use filtration system is highlighted in the Downstream Column’s recent article, “Achieving Peak Flexibility – A single-use filtration skid with multiple application capability.” Download the article here for an overview of what makes this product unique and why its perfectly suited for the ever-changing biomanufacturing industry.
As the article points out, expectations for biomanufacturing require downstream systems to be responsive and flexible to meet ever-changing demands and requirements. And the new single-use multipurpose filtration system is designed with this in mind, providing next level flexibility to meet the needs of customers now and as their bioprocesses evolve.
The Agilitech Advantage:

Flexible brand-agnostic design enables use of best-in-class components for every part of the system

Multiple inlets and outlets provide even more flexibility to meet your exact needs

Manual operation to fully automated control with your preferred automation and control platform (DeltaV®, Rockwell Automation®, or other)

Space-saving compact benchtop and floorstanding model with castors for easy moving

Dynamic flow control, volume totalizer, and more features to optimize your bioprocess!
How it works
The physical aspects of the multipurpose filtration skid are the same from one application to the next and the skid is not set up initially to be technically configured to a specific filter. To configure the skid for a specific application, the appropriate filter is first physically connected to the skid, whether a depth filter, virus filter, or sterile filter. Next, parameters such as optimized flow rate and pressure requirements of the filter are configured for the appropriate application. The system can be used for one step of the filtration process and then configured for another step, as needed. Shown here is the floorstanding model configured for depth, virus, and sterile filtration.
Depth Filtration Configuration

Virus Filtration Configuration

Sterile Filtration Configuration

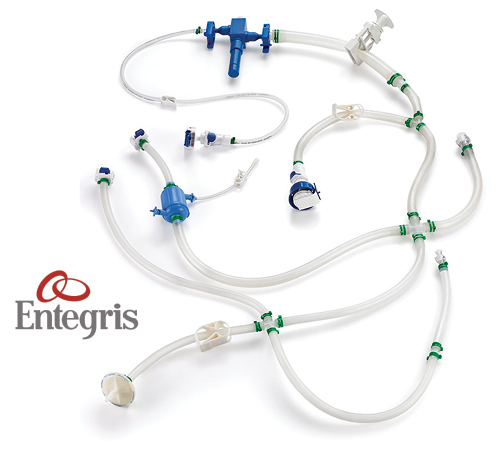
Quality consumables customized for your unique process
The components integrated with Agilitech single-use systems, such as flow path assemblies and 2D/3D bags, are produced by Entegris. With more than 50 years of proven materials science experience behind them, Entegris has developed deep technical expertise to deliver world-class production and exceptional consistency. Entegris flow path assemblies are manufactured to the highest standards and are delivered preassembled, sterilized, and use only optimized components to ensure consistent quality and operational excellence.
- Customized flow path assemblies with integrated sensors and sterile-to-sterile connections—sized to specific batches; accommodate different filters and instrumentation
- 2D Bags—scalable based on batch sizes with customizable inlet and outlet ports
- 3D Bags— scalable to any size up to 2500L with customizable inlet, outlet, sampling, and instrumentation ports
Download product information and other featured content:

Single-use Multipurpose Filtration Systems
1.1 MB

Achieving Peak Flexibility – A single-use filtration skid with multiple application capability
(304 KB)

Agilitech Bioprocess Solutions Overview
Flexible and scalable single-use technologies (867 KB)

Service and support to fit your exact needs
All Agilitech single-use technologies are built to the highest standards and delivered with the documentation you need:
- Standard Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) documentation provided upon delivery; we can also use your specific documentation, if preferred
- Installation Qualification (IQ) and Operational Qualification (OQ) testing and documentation available for use in cGMP production
And if you need more, we will partner with you to develop Performance Qualification (PQ) testing; supplemental documents such as Qualification Project Plan (QPP), Criticality Assessment and Risk Assessment can also be provided.
All Agilitech single-use systems are delivered with a recommended preventative maintenance plan and spare parts list. We also provide maintenance service to ensure pumps, transmitters and all other components function within specification.
You have a vision for your process.
We can help you get there.


